Mastering Surface Forces and Capillary Pressure
Surface Tension and Capillary Pressure: A Deep Dive into Petrophysics
 |
| Mastering Surface Forces: Exploring the Science of Surface Tension and Capillary Pressure on "Petroleum Knowledge Fields" YouTube Channel |
Exploring the "Petroleum Knowledge Fields" YouTube Channel
If you're eager to delve deeper into the fascinating world of surface forces and their applications in the petroleum industry, the "Petroleum Knowledge Fields" YouTube channel is a treasure trove of valuable information. This educational channel is dedicated to sharing insights, experiments, and demonstrations related to surface tension, capillary pressure, and other fundamental concepts in petroleum engineering.
The "Petroleum Knowledge Fields" YouTube channel covers a wide range of topics, from the basics of surface forces to advanced techniques used in the oil and gas industry. The videos are presented in an engaging and accessible manner, making complex scientific concepts easy to understand for both beginners and experts. Whether you're a student, a professional in the field, or simply a curious mind, this channel is a valuable resource for expanding your knowledge and staying up to date with the latest advancements in the field.
Surface Forces Experiments and Demonstrations on the Channel:
One of the highlights of the "Petroleum Knowledge Fields" YouTube channel is the wide array of experiments and demonstrations related to surface forces. These hands-on activities not only make the concepts more tangible and relatable but also provide valuable insights into the practical applications of surface tension and capillary pressure.
For example, one video showcases an experiment where different liquids are poured onto a solid surface and their behavior is observed. By comparing the spreading and beading properties of the liquids, viewers can gain a deeper understanding of the role of surface tension and interfacial tension. Another demonstration involves the use of capillary tubes to investigate the ascent of liquids in narrow channels, simulating real-world scenarios encountered in petroleum reservoirs.
Explanation of content:
- Properties of Petroleum Reservoir Rocks, Surface forces and capillary Pressure Fluid Rock inter interaction. The study of fluid Rock interaction is a fundamental importance to Reservoir engineering. Not only does such interaction influence fluid flow through the reservoir, it also plays a dominant role in the distribution of fluids within the reservoir's poor space, and more importantly, it dictates the maximum amount of a fluid that can be withdrawn from the reservoir. We shall start with the simplest form of such interaction, and then move up to more complex ones: Surface and Interfacial Tension:
- In dealing with multiphase systems, it is necessary to consider the effect of the forces at the interface when two Imiss fluids are in contact. When these two fluids are liquid and gas, the term surface tension is used to describe the forces acting on the interface. When the interface is between two liquids, the acting forces are called interfacial tension. Consider the two emissive fluids: air or gas and water or oil. as shown schematically in following figure. A molecule at the interface, however, has a force acting on it from the air, gas molecules lying immediately above the interface, and from liquid molecules lying below the interface.
- A molecule at the interface, however, has a force acting on it from the air, gas molecules lying immediately above the interface, and from liquid molecules lying below the interface. Resulting forces are unbalanced and give rise to surface tension. The unbalanced attraction force between the molecules creates a membrane likee surface with a measurable tension, I.E surface tension. The following figure can be defined in terms of an emiss fluids, oil and water, In which case, the resulting unbalanced forces at the surface give rise to interfacial tension. The unbalanced attraction force between the molecules creates a membran like surface with a measurable tension such as surface or interfacial tension. Therefore, the interfacial or surface tension has the dimensions of force per unit length usually expressed as D Cm and is usually denoted by the symbol.
- the interfacial tension or surface tension entirely fluid property and not a petroleum Reservoir Rock property. High interfacial tension indicates admissible fluids and low if indicates missible fluids.
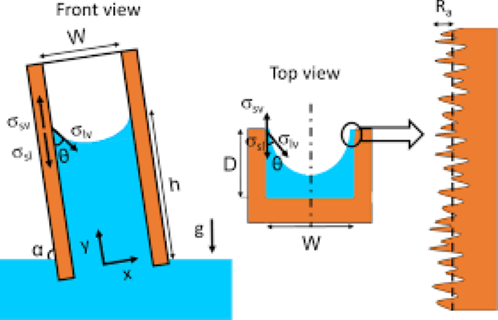
- If a glass capillary tube is placed in a large open vessel containing water, the combination of surface tension and wetability of tube to water will cause water to rise in the tube above the water level in the container outside the tube is shown in following figure. The water will rise in the tube until the total force acting to pull the liquid upward is balanced by the weight of the column of liquid being supported in the tube. Assuming the radius of the capillary tube is R, the total upward Force underscore which holds the liquid up is equal to the force per unit length of Surface Times the total length of surface.
- The total upward Force underscore which holds the liquid up is equal to the force per unit length of Surface Times the total length of surface.
- The total upward Force underscore which holds the liquid up is equal to the force per unit length of Surface Times the total length of surface. The upward force is counteracted by the weight of the water, which is equivalent to a downward force of masstimes acceleration, or the upward force is counteracted by the weight of the water, which is equivalent to a downward force of mass times, acceleration, or because the density of air is negligible in comparison with the density of water.
- Equating the upward force and downward Force where effect of pressure and temperature on interfacial tension. Since gas, oil and water coexist at a variety of high pressure and high temperature conditions in petroleum reservoirs, it is important to understand the effect of these variables on F or St. The variation of If or St with temperature and pressure strongly influences the transport of fluids in a reservoir, and therefore are fundamental to the understanding of the role of interfacial forces in oil recovery.
- Surface tension generally decreases with an increase in pressure and temperature. As temperature increases, the kinetic agitation of the molecules and the tendency of the molecules of fly outward increases, resulting in a decrease in St values. as shown in figure. The effect of pressure on St is also somewhat similar to the temperature effect. The high press gas phase tends to develop Mis ability toward the companion liquid phase, thereby reducing St as pressure increases.
- Capillary pressure: Capillary phenomena occur in pause media when two or more Imiss fluids are present in the the poor space. The difference in the pressure across the interface due to interfacial energy between two Imiss phases results in a curvature of the interface. The rise or depression of fluids in fine board tubes is a result of the surface tension and wetting preference and is called capillarity.
- Capillary Pressure equals pressure of the non-wetting phase Pressure of the wetting phase. There are three types of capillary Pressure Water W Capillary Pressure Gas Wall Capillary Pressure Gas Water Capillary Pressure Applying the mathematical definition of the capillary pressure as expressed by If all the three phases are continuous, then referring to following figure: The pressure of the water phase at Point 2 is equal to the pressure at point for minus the head of the water or the pressure just above the interface at point One represents the pressure of the air and is given by The capillary pressure is zero in a horizontal or plain interface. Therefore, I appreciate your attention. Please subscribe to the channel to get all the new updates.
An overview and specifics regarding the lecture topic
Introduction to Surface Forces:
As an avid science enthusiast, I have always been fascinated by the wonders of the natural world. One particular area of interest that has captivated my attention is the intriguing science of surface forces. These forces play a crucial role in various aspects of our lives, from the way water forms droplets on a leaf to the behavior of oil spills on the ocean's surface. In this article, I will delve into the fascinating world of surface tension, capillary pressure, and surface and interfacial tension, unraveling the science behind these phenomena and exploring their practical applications.
Understanding Surface Tension:
Surface tension is a phenomenon that arises due to the cohesive forces between the molecules at the surface of a liquid. It is what gives rise to the formation of droplets and the ability of certain insects to walk on water. Surface tension can be thought of as a "skin" that forms on the surface of a liquid, causing it to behave as if it were a stretched elastic membrane. This property is responsible for the round shape of droplets, as they minimize their surface area to minimize the energy associated with the surface tension.
To understand surface tension more deeply, let's take a closer look at the molecules that make up a liquid. These molecules are constantly in motion, colliding with each other and bouncing off the walls of their container. However, the molecules at the surface experience a net inward pull due to the cohesive forces with the molecules beneath them. This creates a "pulling" effect, which gives rise to the surface tension. The stronger the intermolecular forces, the higher the surface tension of a liquid.
The Science Behind Capillary Pressure:
Capillary pressure is another fascinating aspect of surface forces that has a profound impact on various natural and industrial processes. It refers to the pressure difference across the interface between a liquid and a solid in a capillary tube or porous medium. This phenomenon is responsible for the rise of liquids in narrow tubes, such as the ascent of water in plants through the xylem vessels.
Capillary pressure arises due to the competition between cohesive forces and adhesive forces. Cohesive forces between the liquid molecules tend to minimize the surface area, while adhesive forces between the liquid and the solid tend to spread the liquid. When the adhesive forces are greater than the cohesive forces, the liquid rises in the capillary tube, defying gravity. On the other hand, if the cohesive forces dominate, the liquid is depressed in the capillary.
Understanding capillary pressure is crucial in fields such as petroleum engineering, where it plays a significant role in the extraction of oil and gas from underground reservoirs. By comprehending the science behind capillary pressure, engineers can optimize the production and recovery of hydrocarbons, leading to more efficient and sustainable practices.
The Role of Surface and Interfacial Tension:
Surface and interfacial tension are closely related concepts that further enhance our understanding of surface forces. Surface tension, as discussed earlier, refers to the cohesive forces between molecules at the surface of a liquid. Interfacial tension, on the other hand, arises at the interface between two immiscible liquids or a liquid and a gas. These phenomena are responsible for the behavior of liquid drops and bubbles, as well as the formation of emulsions.
Surface and interfacial tension are influenced by various factors, including temperature, pressure, and the nature of the liquids involved. They have a profound impact on many industrial processes, such as the production of pharmaceuticals, cosmetics, and food products. By manipulating surface and interfacial tension, scientists and engineers can control the stability, solubility, and dispersion properties of liquids, leading to innovative and efficient products.
Real-life Examples of Surface Forces at Work:
Surface forces are not just confined to the confines of a laboratory or a textbook. In fact, they are at play all around us, shaping the world we live in. Let's explore some real-life examples of surface forces in action.
One of the most fascinating examples is the behavior of water on a lotus leaf. The surface of the leaf is covered with microscopic bumps that trap air pockets. This creates a superhydrophobic surface, causing water droplets to bead up and roll off the leaf, carrying dirt and debris with them. This self-cleaning mechanism is a result of the high surface tension of water and the low surface energy of the leaf, which repels the water molecules.
Another intriguing example is the formation of soap bubbles. Soap is a surfactant that reduces the surface tension of water, allowing it to spread and form a thin film. When air is trapped within this film, it creates a bubble. The spherical shape of the bubble is a result of the surface tension, which tries to minimize the surface area of the film. The vibrant colors observed on soap bubbles are due to the interference and diffraction of light as it passes through the thin film.
How to Apply Surface Forces Knowledge in Industrial Applications:
The knowledge of surface forces, including surface tension, capillary pressure, and surface and interfacial tension, has far-reaching implications in various industrial applications. Understanding these concepts can lead to innovative solutions and improved processes in fields such as petroleum engineering, material science, pharmaceuticals, and many more.
In the petroleum industry, for instance, the understanding of surface forces is essential for optimizing oil recovery from reservoirs. By manipulating capillary pressure and interfacial tension, engineers can enhance the displacement of oil by injecting water or other fluids into the reservoir. This leads to improved production rates and higher overall recovery factors.
In the field of material science, surface forces play a crucial role in the development of coatings, adhesives, and surface treatments. By tailoring the surface tension and interfacial properties of materials, scientists can create superhydrophobic surfaces, self-cleaning coatings, and bioadhesive materials. These advancements have numerous applications, ranging from consumer products to medical devices and aerospace technologies.
Conclusion:
Surface forces encompass a captivating realm of science that influences our daily lives in more ways than we can imagine. From the behavior of water droplets to the extraction of oil from underground reservoirs, surface tension, capillary pressure, and surface and interfacial tension shape the world around us. By exploring the "Petroleum Knowledge Fields" YouTube channel and delving into the science behind surface forces, we can gain a deeper understanding of these phenomena and apply our knowledge in various industrial applications. So, let's embark on this journey of discovery and mastery, unraveling the secrets of surface forces one experiment at a time.
CTA:
Subscribe to the "Petroleum Knowledge Fields" YouTube channel and join the community of surface forces enthusiasts as we explore the fascinating world of science together!


Comments