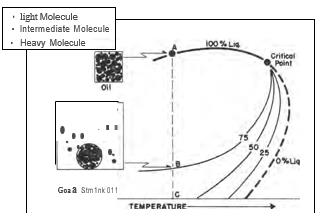
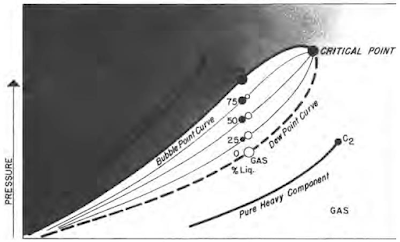
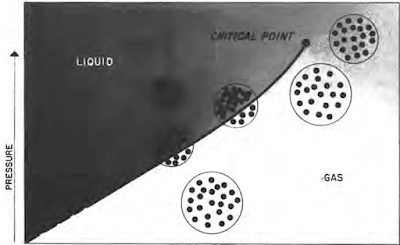
Retrograde Condensate Gas
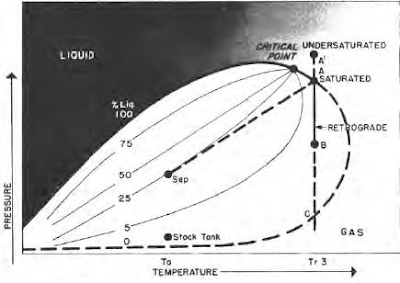
Understanding the Key Characteristics of Oil and Gas Some hydrocarbon mixtures exist naturally above their critical temperature as gas condensates. When pressure is decreased on these mixtures, instead of expanding (if a gas) or vaporizing (if a liquid) as might be expected, they tend to condense. Conversely, when pressure is increased, they vaporize instead of condensing. The process is illustrated by temperature condition Tr3 in Fig. 41, and the gas condensate curve in the next figure. This process is caused by forces acting on molecules of unlike sizes and depends upon a balance of these forces, as illustrated in the figure. Phase diagram of retrograde condensate gas Equilibrium retrograde behavior of condensate gas Normal vaporization and condensation, on the other hand, depend more upon balance between molecular forces of like-size component molecules. As pressure drops (at constant temperature) below dew-point pressure (A), the attraction between light and heavy component m...