Equilibrium Separation
Characteristics of Oil and Gas
Equilibrium Separation
Low Shrinkage Oils
A given volume of oil existing at its bubble point at reservoir temperature is considered to be saturated with gas at the given temperature and pressure conditions. Thus, the term "saturation pressure" is synonymous with bubble-point pressure at a given temperature. A decrease in pressure will cause the original sample to change into two phases as shown in the next figure. The physical change is evidenced as gas being liberated from the liquid.
It is a common misbelief that a certain amount of gas is dissolved in oil and that a pressure drop results in the gas coming out of solution. Actually, the first gas liberated is composed principally of the lightest components (methane, ethane and propane) because these components possess the highest molecular energy and the lowest molecular attraction for other molecules.
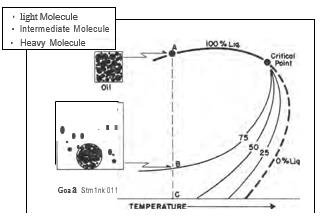 |
| Equilibrium vaporization of low shrinkage oil. |
For low shrinkage oil, there is a somewhat uniform change in shrinkage as pressure drops through the high and intermediate pressure range. This shrinkage occurs principally as a result of volumetric loss of light materials. It increases rapidly, however, through the low-pressure range. This shrinkage occurs principally through volumetric loss of intermediate and heavy material from the remaining liquid. Shrinkage characteristics of this range of pressures are extremely significant because surface separation of oil from gas occurs under these conditions.
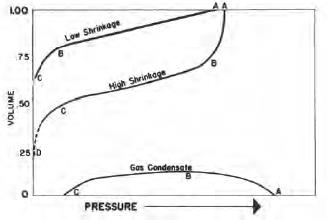 |
| Equilibrium shrinkage of hydrocarbon liquids |
High Shrinkage Oils
Certain oils are known as high shrinkage oils because their shrinkage with pressure reduction is greater than normal. The term "high shrinkage" is qualitative because there is no distinct set of conditions by which low and high shrinkage oils may be classified. Higher shrinkage is usually brought about because of the existence of greater quantities of intermediate components or lesser quantities of heavy components in the mixture.
Changes in oil shrinkage by vaporization of material through pressure reduction is illustrated by temperature condition Tr2 in the phase diagram and the high shrinkage curve.
Behavior of high shrinkage oil in the high pressure range is different from that of low shrinkage oil. As pressure drops slightly from saturation pressure (A to B), not only do the light component molecules leave solution, but a large quantity of intermediates also leave solution to form gas. Actually, it is the presence of this large quantity of intermediate components that makes a high shrinkage oil. High shrinkage of the oil from A to B is caused not only by attraction of intermediate component liquid molecules to closely spaced, light component gas molecules, but also by the normally high kinetic energy of the intermediate component liquid molecules.
As pressure drops further (B to C), the attraction for intermediate component liquid molecules by light component gas molecules decreases because of an increase in the distance separating them;
therefore, there is a tendency toward greater attraction between the remaining liquid intermediates to liquid heavies, which prevents further rapid vaporization throughout the middle pressure range. Vaporizing tendency of heavy component liquid molecules increases through the low-pressure range (C to D), just as in the case of low shrinkage oils, although to a greater degree because of the greater quantity of intermediates held by attraction to the heavy liquid molecules until the low-pressure condition is reached. High shrinkage oils behave similarly to low shrinkage oils in the low pressure range, except that shrinkage is much greater. Shrinkage characteristics of high shrinkage oils are, therefore, of great significance in surface separation problems.
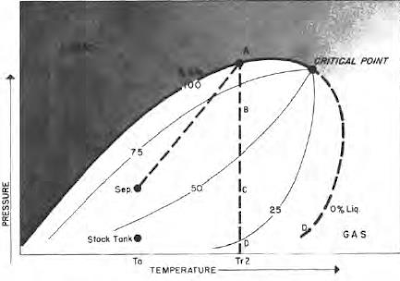 |
| Phase Diagram of high shrinkage oil |
keywords: Shrinkage, Vaporization, equilibrium, separation.


Comments