Changes in Phases
Characteristics of Oil and Gas
Changes in Petroleum Phases
If pressure is increased, molecules are forced closer together so that gas will be compressed or changed to a liquid. However, as pressure is decreased, the reverse occurs; gas expands and liquid tends to vaporize to gas. The molecules in the latter case are thrown apart by their own kinetic energy and molecular repulsion. These phase changes caused by changes in pressure are termed normal or regular behavior.
If the molecules are smaller, as in the case of methane and ethane, there is less attraction between molecules and greater tendency for them to be thrown apart by their kinetic energy into gas; whereas, if molecules are larger, as in hexane and heptane, they tend to be attracted together into the liquid rather than thrown into the gaseous state by their kinetic energies. As the temperature of the compounds increases, kinetic energy increases. The tendency then is for all molecules in the liquid state to be thrown into the gaseous state and for gases to expand. However, if temperature is decreased, the kinetic energy decreases, and all molecules (even the lighter molecules) tend to be attracted together into a liquid state and even frozen into the solid state if the temperature is low enough. This behavior is also considered normal or regular.
Pure Hydrocarbons
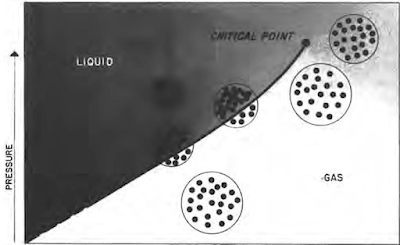
For a single or pure hydrocarbon such as propane, butane or pentane, there is a given pressure for every temperature at which the hydrocarbon can exist both as a liquid and a gas. This is demonstrated in Figure. Under these conditions, the forces which tend to pull the molecules closer together (pressure and attractive forces) balance the kinetic energy which tends to throw them apart. If pressure is increased without a temperature change, the gas molecules are forced closer together and the attractive forces between molecules are thus increased. The forces then tending to pull molecules together are greater than the kinetic energy, and the molecules condense to a liquid state.
However, if pressure is decreased without a temperature change, the distance between gas molecules is increased, and the attractive forces are thus decreased. Forces tending to pull the molecules together are then less than the kinetic energy, and the molecules disperse into a gas.
Actually, pressure results from the molecular bombardment of the containing vessel and liquid surface. The increase in volume tends to reduce the pressure by increasing the distance molecules must move to strike the container. As temperature increases, kinetic energy is increased; higher pressures are required for existence of the balanced conditions at which the two phases can exist simultaneously. The curve plotted through the pressure-temperature points where two phases exist is called the "vapor-pressure curve". There is a temperature above which the material will not exist in two phases regardless of the pressure. This is called the "critical point", and temperature and pressure at this point are called "critical temperature" and "critical pressure".
It is common practice to consider the material as a gas when it exists at temperature and pressure conditions below the vapor-pressure curve, and as a liquid above the vapor-pressure curve. Such a definition obviously is confusing because ranges of temperature and pressure exist in which the material can be classified as either liquid or gas. These ranges are shown in the upper right-hand portion of Figure. In these ranges, the temperature is so great that attractive forces between the molecules are not sufficiently great to permit them to coalesce to a liquid phase because of the higher kinetic energy of molecules at the high temperature condition. Under these temperature conditions, an increase in pressure causes the molecules to move together uniformly as pressure is increased. This confusion is minimized when working with reservoirs because reservoir temperature (and thus the temperature of the hydrocarbons in the reservoir) usually remains constant; therefore, only pressure and volume are usually altered to an appreciable degree in the reservoir during production.


Comments