Phases of Petroleum
Characteristics of Oil and Gas
Phases of Petroleum Molecular Behavior
Phases of Petroleum
Because of a man's environment of earth, water and air, it could well have been that his first scientific observations were that matter existed in three states or forms; solid, liquid and gas. Generally, it is found that all substances may exist in any of the three forms which have been termed phases of matter.
Whether a substance exists in a solid, liquid or gas phase will be determined by temperature and pressure conditions acting on the substance. It is well known that steam can be changed to water by lowering its temperature and water can be changed into ice by further lowering its temperature. Hydrocarbon compounds, either individually or in mixtures, will change their state or phase in the same way by changing their temperature and pressure. The resulting change is called "phase behavior".
Heavy hydrocarbons, including paraffin and tars, sometimes form naturally as solids in the reservoir and present problems in oil production operations; however, this does not often happen, and such problems will not be discussed herein. The discusses will be limited to the behavior of hydrocarbons as they change from gas to liquid and liquid to gas and the operating problems and their control caused by such changes.
Molecular Behavior
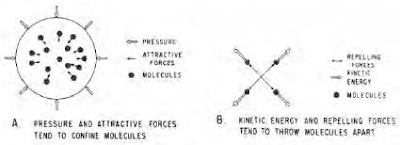
Hydrocarbons behave peculiarly when their pressure and temperature are changed. This behavior is best explained by the behavior of the individual molecules making up the mixture. Four physical factors are important in governing the behavior of hydrocarbon matter. These are (1) pressure, (2) molecular attraction, (3) kinetic energy (molecular motion associated with temperature) and (4) molecular repulsion (Figure).
Pressure and molecular attraction tend to confine molecules or pull them together so that the greater the value of these forces, the greater the tendency for the material to become more dense, as is the case when gases become liquid.
 | |
|
Kinetic energy, or molecular motion, increases as temperature increases so that the greater the temperature of a material, the greater the tendency for the material to be thrown apart, and thus decreases in density or changes from a liquid to a gas (or for a gas to expand).
When molecules get so close together that their electronic fields overlap, a repelling force is present that tends to increase the resistance of the material to further compression. When hydrocarbon material appears to be at rest (not expanding, contracting in volume or changing state), the forces tending to confine the molecules balance the forces tending to throw them apart, and the material is considered to be in equilibrium.
Changes in Phases
If pressure is increased, molecules are forced closer together so that gas will be compressed or changed to a liquid. However, as pressure is decreased, the reverse occurs; gas expands and liquid tends to vaporize to gas. The molecules in the latter case are thrown apart by their own kinetic energy and molecular repulsion. These phase changes caused by changes in pressure are termed normal or regular behavior.
If the molecules are smaller, as in the case of methane and ethane, there is less attraction between molecules and greater tendency for them to be thrown apart by their kinetic energy into gas; whereas, if molecules are larger, as in hexane and heptane, they tend to be attracted together into the liquid rather than thrown into the gaseous state by their kinetic energies. As the temperature of the compounds increases, kinetic energy increases. The tendency then is for all molecules in the liquid state to be thrown into the gaseous state and for gases to expand. However, if temperature is decreased, the kinetic energy decreases, and all molecules (even the lighter molecules) tend to be attracted together into a liquid state and even frozen into the solid state if the temperature is low enough. This behavior is also considered normal or regular.
keywords: molecules, behavior, phases.


Comments